
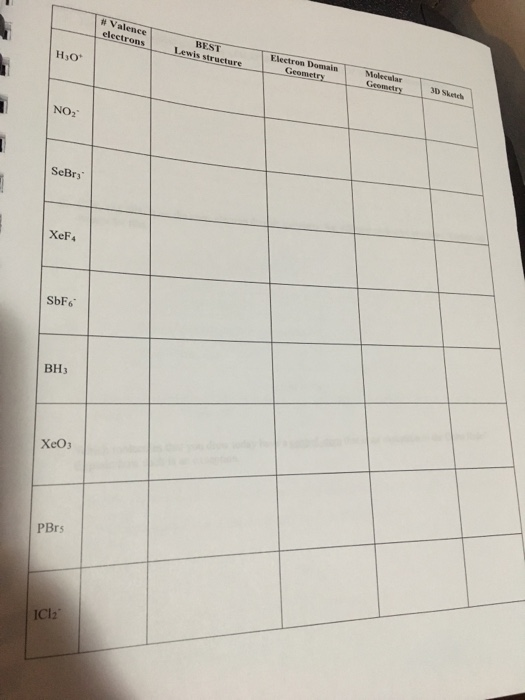
Bonding in the series of compounds P4, P4O6, P4O10, N4(CH2)6 (hexamethylenetetramine) and (CH)4(CH2)6 (adamantane) is discussed in the context of the isolobal model.Įlectron localization function fragment formalism isolobal topological analysis two-electron three-center bond valence-shell electron-pair repulsion. This unifying concept as well as the calculations we have carried out fully agree and also give support to earlier ideas developed by Bragg and Bent, among other authors. In this model the O atoms would play the same role as the formally present O(2-) anions in the `so-called' ionic solids, such as in the skeletons of aluminate and silicate polyanions, thereby connecting molecular and solid-state chemistry as formulated by the `fragment formalism' or the `molecular unit-cell approach'. According to VSEPR, the molecular geometry or shape of BH 3 will appear as a Trigonal planar.

Because it has 3 regions of electron density around the Boron (B) central atom, all are the bonding regions. On the contrary, in the angular geometry the same O atoms form two Si-O bonds and its lone pairs mimic the geometry of the -CH2- group. The molecular geometry of BH 3 is trigonal planar. At the same time, the three oxygen lone pairs mirror the three C-H and B-H bonds, respectively. Is the sum of the formal charge equal to the net charge YES neutral compounds have a net charge of zero d. is the formal charge on all atoms equal to zero YES c. S1 : N2H 4 is pyramidal about each N atom (3) BH3. Draw the most likely Lewis Dot Structure: NO NO b. To understand the hybridization, polarity and molecular geometry of the Ozone molecule. Chemistry Chemistry questions and answers Problem4: Use VSEPR to predict the molecular geometry of BH3.

IUPAC name for BH3 is borane which is also known as trihydrido borane. The hybridization of boron in BH 3 is sp 2. The ELF analysis confirms the idea that the O atom, in the linear geometry of (H3C)3-Si-O-Si-(CH3)3, is isolobal with the isoelectronic -CH3(+)- and -BH3- groups, the bonding in the Si-O-Si group being described as a two-electron, three-center (2e, 3c) bond. The electrons in the outer shell of an atom are involved in bonding. BH3 (borane) is comes under natural products which is originated from Erysimum inconspicuum. The electron geometry of BH 3 is also Trigonal planar as its central atom has 3 regions of electron density. A topological analysis of the electron localization function (ELF) of a molecule of hexamethyldisiloxane, (H3C)3-Si-O-Si-(CH3)3, has been carried out, drawing a consistent picture of Si-O-Si bonding both in the linear and angular geometries.


 0 kommentar(er)
0 kommentar(er)
